The diverse early embryonic development of vertebrates
refutes their common ancestry
The characteristic features of vertebrates, notably their backbone with accompanying spinal cord and gut, have of course been known for a long time. Embryological studies in the 19th century revealed that the various classes of vertebrates (such as cartilaginous and teleost fish, amphibians, reptiles, birds, mammals), although having very different adult forms, in the course of early embryonic development look quite similar. More generally, there is an embryonic phylotypic stage which is common to members of a phylum; in vertebrates this is sometimes called the pharyngula stage. Karl von Baer (1792-1876) summed this up in his laws of embryology which included that within a group of organisms (such as the vertebrates) in the course of their embryonic development the common or general characters arise first, and then the features that distinguish the different classes arise through subsequent specialisation.
Von Baer saw these similarities merely as reflecting that vertebrates have a common body-plan. But Darwin of course saw similarities of embryonic development as important supporting evidence for his theory of evolution: if the different classes of vertebrates had descended from a common vertebrate ancestor then they would develop embryonically in comparable (or homologous) ways. Ernst Haeckel (1834-1919) produced drawings (Figure 1) which – for the purpose of supporting Darwin's theory, and his own of 'ontogeny recapitulates phylogeny' – exaggerate the similarities of the early embryos of several vertebrate classes. Despite this well-known misrepresentation [1], the figures have been widely reproduced, even in recent textbooks etc. about evolution. And no doubt fostered by the widespread dissemination of Haeckel’s drawings, this is the popular view – that embryology, especially of the vertebrates, clearly supports that they are derived from a common ancestor, and hence clearly supports evolution.
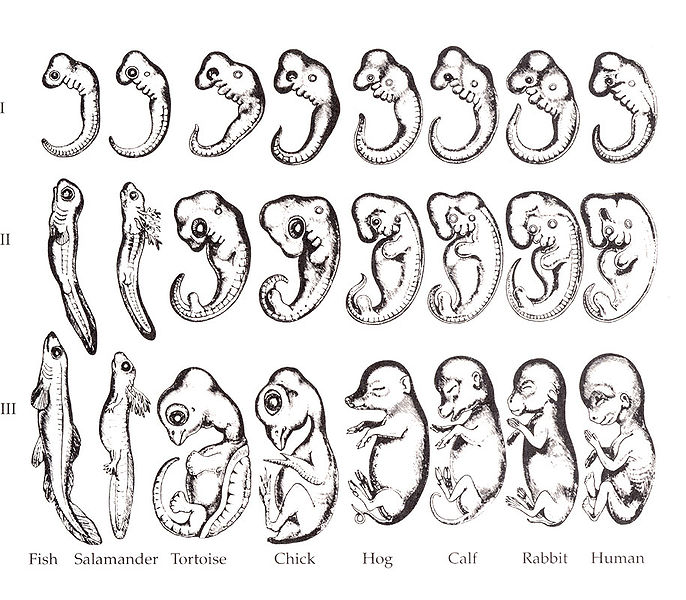
Figure 1. Haeckel's embryos. [a]
However, it has been known since at least the early 20th century that there are some significant exceptions – where vertebrates do not have similar embryonic development, even in the early stages, in fact are substantially different. I came across indications of this while writing Evolution under the microscope, such as Homology: an unsolved problem (1971) by Gavin de Beer (1899-1972) who gave this as one example:
Structures as obviously homologous as the alimentary canal in all vertebrates can be formed from the roof of the embryonic gut cavity (sharks), floor (lampreys, newts), roof and floor (frogs), or from the lower layer of the embryonic disc, the blastoderm, that floats on the top of heavily yolked eggs (reptiles, birds). [2]
So I planned that one day I would take a proper look. It’s quite a substantial task because it involves acquiring a reasonable knowledge of how different classes of vertebrates develop. But it has been worth the effort! I have been surprised by how substantial are the differences of early embryonic development in different vertebrate classes; the differences are much more significant and radical than any inaccuracies in Heackel's drawings. As I have written in About me, I had believed what I had been taught – that the embryonic development of vertebrates is similar and is evidence of common ancestry and evolution – but have found that in fact the early embryonic development of different groups of vertebrates is very diverse, and this constitutes clear evidence against their common ancestry.
The evidence is extensive, and one of the difficulties is how to present it in an accessible way. I have decided to put all of the background material in the section on embryology, and kept the comparisons and conclusions drawn from those comparisons in this section on homology. This page presents just an overview, to try to give some indication of how substantial and radical are the differences; more detailed comparisons of the different stages of development are given in subsequent pages.

Outline of embryonic development
Overview comparison of vertebrate embryogenesis
Outline of early embryonic development
To make the following table comprehensible for those not familiar with embryonic development, this is a very brief outline of the early stages:
- Fertilisation. The result of fertilisation of an egg by a sperm is a zygote, which is a single cell.
- Cleavage. The first stage of development is cleavage – successive divisions of the zygote – to produce a population of cells.
- Blastula. Initially the cells are similar; but typically by the time there are a few hundred cells, they organise into a structure called a blastula (in mammals called a blastocyst) which has differentiated into at least two, sometimes three or more, distinct groups of cells.
- Gastrulation. Gastrulation is the overall term to refer to the various processes whereby the cells of the blastula rearrange and differentiate to produce the three germ layers:
– an outer layer of ectoderm,
– an inner layer of endoderm, and
– mesoderm in-between,
from which all of the body’s tissues develop. - Neural tube. Ectoderm along the midline of the embryo’s back develops into the neural tube, which is the precursor of the spinal cord.
- Mesoderm.
– Mesoderm along the centerline forms the notochord;
– either side of this forms the somites (precursors of the vertebrae); and
– either side of those it forms various structures including the central body cavity (coelom). - Gut. Endoderm develops into the gut and associated digestive organs.
- Extraembryonic membranes. Reptiles, birds and mammals have substantial membranes which are not part of the embryo, but have key roles in its development: amnion (which gives these classes of vertebrate the collective term amniotes), chorion, and allantois.
Phylotypic stage and common ancestry
Once the above stages have taken place, the various embryos have more-or-less reached a more-or-less similar ‘phylotypic’ stage. (There is debate about what stage can best be regarded as the vertebrate phylotypic stage, or even to what extent such a stage exists.) Whilst there are differences between them, they all share features such as an anterior-posterior axis, a dorsal notochord and neural tube, flanked by paired somites, a primitive gut surrounded by endoderm, and a coelomic cavity.
In view of the considerable similarities during this phase of the various vertebrate classes it is not at all surprising that it led to the idea of a phylotypic stage; and this concept may still be appropriate.
However, whereas the existence of a vertebrate phylotypic stage is consistent with the possibility that the vertebrates have evolved from a common vertebrate ancestor, this second conclusion can no longer be sustained in the light of what we now know about the very diverse ways in which the phylotypic stage arises in different groups of vertebrates.

Outline of embryonic development
Overview comparison of vertebrate embryogenesis
Overview of some key differences of early embryonic development of vertebrates
The following table gives an overview of some of the key differences of early embryonic development of different classes of vertebrates.
gastrulation
neurulation
gut formation
extraembryonic
membranes
blastula
gastrulation
neurulation
gut formation
extraembryonic
membranes
blastula
gastrulation
neurulation
gut formation
extraembryonic
membranes
blastula
gastrulation
neurulation
gut formation
extraembryonic
membranes
blastula
gastrulation
neurulation
gut formation
extraembryonic membranes
Figure 2. A comparison of early vertebrate embryonic development.
Some key comparisons
As mentioned above, it is common for e.g. textbooks on evolution to emphasise that at the phylotypic stage the embryos of different vertebrate classes look similar, and present this as evidence of common ancestry. But it is clear from the above table that in the preceding stages the different types of embryos look very different. And these readily apparent differences are reinforced by the less obvious but even more significant very diverse morphological processes that shape the early embryo.
The following summarises some key differences; the headings are links to pages that give more detail.
Cleavage
What is so striking – and so compelling as evidence against common ancestry – is that the embryonic developments are so very different right from the outset. From the very earliest stage – cleavage – it occurs in substantially different ways.
1. To begin with, there is the very substantial difference between holoblastic and meroblastic cleavage, which is usually ‘explained’ in terms of the yolk content of the egg:
It is presumed that the vertebrate ancestors employed holoblastic cleavage. It is then argued that in various vertebrate lineages yolk content increased because it provides nutrient for the growing embryo. Cell divisions cannot penetrate the increased yolk, so this results in cleavage becoming meroblastic. And it is inferred that this transition occurred independently five times in the course of vertebrate evolution.
However, this is typical of evolutionary thinking: An evolutionary origin of vertebrates from a common ancestor is assumed; then, because of the distribution of holoblastic and meroblastic cleavage in the cladistic classification of vertebrates, it is concluded that meroblastic cleavage must have evolved from holoblastic however many times were required (with reversions if necessary) to account for its cladistic distribution. But little or no consideration is given to the genetic and molecular implications of such transitions.
2. In addition, there are some significant features about cleavage in some vertebrate groups:
Teleosts
- The first 6 cleavage divisions are very stereotypical, resulting in a regular 3-dimensional array of 2 x 4 x 8 cells.
Amphibians
- The first cell division is preceded by rotation of the zygote’s cortex.
- A blastocoel begins in the course of the first cell division (rather than by the more usual segregation of cells at a later stage).
Mammals
- Cleavage is 'rotational' – the two second cell divisions are in different planes – which is unique in vertebrates.
Blastula
The blastulas of the various vertebrate classes are so different from each other that it is difficult to group them or summarise the differences, so I refer readers to the main page on blastulas. It is particularly significant that there are substantial differences even between those parts of the blastula that will develop into the actual embryo:
Chondrichthyans
It is a one-cell thick epithelial layer, forming the upper surface of the blastula.
Teleosts
It is a multiple-cell layer, not epithelial because it is beneath an enveloping layer.
Amphibians
It is the whole of the blastula, comprising the multi-layered dome of the animal hemisphere and the mass of cells in the vegetal hemisphere.
Reptiles and birds
It is the upper surface of the blastula, comprising a single-cell thick epithelial layer, overlying the hypoblast.
Mammals
It is part of the inner cell mass, within the outer trophoblast.
Hypoblast
The blastulas of amniotes have a distinct layer of cells known as the hypoblast, which is absent from anamniotes. In some anamniotes, superficial cells that have internalised in the course of gastrulation may be called ‘hypoblast’, but these are different from the pre-gastrulation hypoblast of amniotes.
Gastrulation
Gastrulation is a key stage of embryonic development because it leads to the establishment of the three germ layers – ectoderm, mesoderm and endoderm – which are characterisitic of vertebrates, and from which all of their body’s tissues are derived. So from an evolutionary perspective we would expect gastrulation to be ‘conserved’, i.e. substantially the same throughout the vertebrates. Yet for almost all of these major classes of vertebrates, the mechanism of gastrulation is substantially different from any of the others:
Chondrichthyans – by cells rolling over an overhang of the posterior of the blastula.
Teleosts – by involution around all of the edges of the blastoderm as it spreads around the yolk.
Amphibians – by involution through an anular blastopore.
Reptiles – by involution through a blastopore.
Birds and mammals – by cells ingressing through a primitive streak.
I shall make a particular comment about gastrulation in reptiles. It has become popular in recent years to see birds not just as having evolved from reptiles (as mammals are said to have done), but even that birds are a subgroup of reptiles, to the extent that traditional reptiles are increasingly referred to as non-avian reptiles. This view is mistakenly supported by the false assertion – even in recent texts about evolution – that gastrulation in reptiles is via a primitive streak, as in birds and mammals. However, it has been known since the 19th century that gastrulation in reptiles is by involution through a blastopore, completely different from ingression through a primitive streak. This is true for all reptiles that have been examined; there is no example known of reptile gastrulation via a primitive streak.
First and foremost, this substantial difference of a key stage of early embryonic development clearly demarcates birds from reptiles, and even undermines the view that birds evolved from reptiles. Unfortunately, it is also an example of how evidence that is inconsistent with the prevailing evolutionary view is often ignored or even suppressed.
Sources of the germ layers
In most cases, all three germ layers arise from a common layer of cells of the blastula; although, as noted above, there are substantial differences in the location and nature of this common layer between the different classes of vertebrates. However:
- The amphibian endoderm is a major exception to this: whereas the ectoderm arises from the dome of cells of the animal hemisphere, the endoderm is formed from the cells of the vegetal hemisphere.
- The areas of cells becoming endoderm (other than in amphibians) and mesoderm, or remaining as ectoderm, are the opposite way round between anamniotes and amniotes.
- In anamniotes, in the course of gastrulation, cells at the edge of the blastula fold or involute beneath the surface layer and become mesoderm or endoderm (except in the case of amphibians), and the cells which remain to become ectoderm are from the middle of the blastula.
- In contrast, in amniotes gastrulation involves the involution (in the case of reptiles) or ingression (in birds and mammals) of cells from the middle of the epiblast, and it is cells from the edge of the epiblast that remain to become ectoderm.
So it’s not just that gastrulation – the embryonic stage in which the germ layers arise – occurs by different mechanisms, but there are also differences in the sources of germ layer tissues – which clearly is non-homologous.
Neural tube
The vertebrate neural tube forms embryonically in two very different ways:
- In amniotes and chondrichthyans, it is by the fusion of folds which arise from the ectoderm.
One aspect of this is that the epithelial nature of the starting ectoderm is retained throughout the process, and inherited by the inner surface of the neural tube lumen.
- In teleosts and amphibians it is by cavitation within a mass of cells that forms by convergence of the ectoderm.
The mass of cells are mesenchymal and a mesenchymal-to-epithelial transition must occur to generate the epithelial cells of the neural tube lumen.
And there are substantial differences between the teleosts and amphibians, for example:- Formation of the lumen in teleosts involves mirror-symmetric cell division which means that cells from both sides of the neural tube are derived from both sides of the neural plate.
- In amphibians the initial ectoderm comprises two layers of cells, both of which are reinstated after formation of the neural tube.
(See the comparison of neural tube formation for comments on 'primary' and 'secondary' neurulation.)
Somitogenesis
In all vertebrates the somites arise from the paraxial mesoderm which forms as a double layer either side of the neural tube during the course of gastrulation; however:
In most vertebrates,
- the somites form by budding off from both layers of mesoderm,
- a process which involves epithelialisation of some of the mesenchymal mesodermal cells.
Whereas in amphibians,
- the somites form from only the inner layer of mesoderm,
- it does not involve epithelialisation, but
- it does involve the cells of the inner mesoderm rotating through 90°.
Formation of the gut
The vertebrate digestive tract is formed in three completely different ways:
- invagination of the outer layer of cells (amphibians),
- fusion of endoderm folds (chondrichthyans and amniotes), and
- cavitation within the endoderm (teleosts).
Amniote extraembryonic membranes
The extraembryonic membranes are the yolk sac, amnion, chorion and allantois which occur in reptiles, birds and mammals. They develop embryonically in two radically different ways:
- In most amniotes they develop after gastrulation by the formation of folds of ectoderm/mesoderm or endoderm/mesoderm, which then fuse above (dorsal) or below (ventral) the embryo respectively.
- In primates they develop before gastrulation by cavitation within parts of the blastocyst.
This is one of the most striking anomalies from an evolutionary perspective:
- the morphological differences between these two developmental mechanisms are so substantial;
- to change from the usual fusion of post-gastrulation folds to cavitation within the pre-gastrulation blastula, would entail changes at such an early stage of development; and
- would need to have occurred within such a recently evolved group as the placental mammals.
Vertebrae
Formation of the vertebrae is usually regarded as occurring after the phylotypic stage; but I include it here for completeness. Importantly, it is not just another example of how early embryonic development is substantiually different in different groups of vertebrates, but of course the formation of vertebrae is especially significant because they are the primary defining feature of vertebrates – yet they form embryonically in very different ways:
- tetrapods – entirely from the sclerotome of somites, via a perichordal tube around the notochord, cartilagenous vertebrae which then ossify, and including resegmentation of the somite/sclerotome;
- chondrichthyans – entirely from the sclerotome of somites, via paired blocks of cartilage (arcualia) which form either side of the notochord, including resegmentation of the somite/sclerotome;
- teleosts – primarily from the notochord, including by direct ossification rather than via cartilage precursors, and no resegmentation.

Outline of embryonic development
Overview of different vertebrate embryogenesis
Features distinct to each class
Distinctive embryonic features of major vertebrate groups
For every major group of vertebrates, its early embryonic development is distinct from that of others in at least one substantial feature.
Chondrichthyans
- Gastrulation is by cells rolling over a posterior overhang of the blastula.
- Vertebrae form via cartilaginous arcualia.
Teleosts
- The blastula has an enveloping layer which has a major role in emboly and gastrulation, but does not become part of the embryo.
- Gastrulation is by involution all around the edge of the blastula.
- The neural tube forms by convergence and then cavitation of ectoderm.
- The digestive tract forms by cavitation of endoderm.
- Vertebrae form from and by ossification around the notochord.
Amphibians
- Cortical rotation occurs before cleavage.
- The blastocoel begins during the first cell division.
- The digestive tract forms by invagination of the blastula.
- Neurulation involves formation of a neural groove, but the lumen forms by cavitation.
- The somites form from only the inner (splanchnic) layer of mesoderm.
Amniotes
- Their blastula includes a hypoblast.
- They have extraembryonic membranes.
Reptiles
- Gastrulation is via a blastopore through the embryonic disc.
Birds
- The hypoblast forms by cells spreading from the posterior margin of the hypoblast.
Mammals
- Rotational cleavage.
- Formation of the blastocoel involves compaction of the cells of the morula.
- The blastocyst comprises an inner cell mass of epiblast and hypoblast within a trophoblast.
- Extraembryonic membranes arise by cavitation of parts of the blastocyst (in primates).
The developmental hourglass — the evolutionary response to diverse early embryonic development
The diverse embryonic development after the phylotypic stage is of course not surprising, because how else could the adult forms be different. But it was expected that the development leading up to to phylotypic stage would be substantially similar:
As first pointed out by von Baer in the 1820s, animals within a phylum, such as the vertebrates, share a common body plan, and in their development share a phylotypic stage in which the body plan elements characteristic of the phylum appear. The process of early development from the egg to the phylotypic stage should be at least as conserved as the pattern of the phylotypic stage. One might reasonably expect mechanisms of early development to be especially resistant to modification because all subsequent development derives from early processes. Traditionally, features of early development and conserved larval stages, even between phyla, have been regarded as strong homologous characters for the inference of phylogeny. [3]
So what is the evolutionary response to the diverse early embryonic development of different groups of vertebrates?
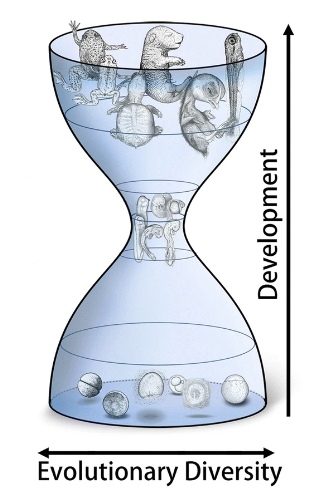
Figure 3. Embryonic development
depicted as an hourglass. [c]
On one hand, the diversity of early embryonic development in vertebrates is still not particularly well known even among professional biologists, many of whom continue to point to the similarities of the phylotypic stage as evidence of their common ancestry.
On the other hand, some of those working in embryology are aware of the diversity — what is their response?
It was in 1994 that Denis Duboule [4], recognising the diversity of embryonic development before and after the relatively similar phylotypic stage, first described embryonic development as an hourglass, and the concept has been adopted quite widely. Unfortunately, having assigned an appropriate term to describe the phenomenon, many biologists now write as if it were also an adequate explanation – which clearly it isn’t. A recent paper on the developmental hourglass comments:
Moreover, it would be interesting to know how animals could have tolerated changes in early developmental stages while conserving the phylotypic period. [5]
But behind this question is the presumption that, despite their diverse early development, the vertebrates have evolved from a common ancestor, and that their embryonic processes must have changed – even though it contradicts the commonsense conclusion (expressed by Raff, above) that:
One might reasonably expect mechanisms of early development to be especially resistant to modification because all subsequent development derives from early processes. [3]
Conclusions
1. At the very least, it is clear that the popular view, that supposed evolutionary relationships of vertebrates are reflected in embryonic development, is false.
It is disingenuous of evolutionary biologists to point to the similarities of the vertebrate phylotypic stage as evidence of common ancestry, because putative evolutionary implications of the similarities of this stage are undermined by the very different ways this stage arises.
2. It is especially significant that different parts of the blastula develop into the embryo. This is because the cardinal criterion of homologues is that they be the same tissue, albeit modified in different ways. Hence, where the embryos of different vertebrate classes develop from different parts of the blastula, it is clear that the resulting embryos are not homologous. And this conclusion applies despite the similar appearance of the subsequent phylotypic stages.
3. The straightforward conclusion to draw from the diversity of early embryonic development of the vertebrates is that it shows they have not evolved from a common vertebrate ancestor.
This conclusion can be avoided only if there are realistic explanations for how such drastic diversity in early development from a common ancestor might have occurred in an evolutionary way i.e. via small changes that:
- were opportunistic or undirected (because evolutionary mechanisms, including natural selection, do not have foresight);
- had a realistic probability of occurring (e.g. by random mutations); and
- at least most of which must have offered significant advantage that could be favoured by natural selection.
Further, such explanations cannot be simplistically based solely on morphological changes i.e. without regard to what we now know about how embryonic development is implemented via the orchestrated action of many interdependent genes.
For example, consider the drastic changes to early embryonic development that would be required to change the sources of the blastula that become the embryo: These would need to have been achieved solely through randomly occurring changes to genes and control sequences, all of which must have had a reasonable chance of happening, and of offering some advantage that could be favoured by natural selection.
Yet, of course, all of the mutations we know of that affect early embryonic development, far from being advantageous, are detrimental if not fatal.
Notes
1. Michael Richardson (1995), Heterochrony and the Phylotypic Period, Developmental Biology 172 pp 412-421, quotations from p418.
2. Gavin de Beer (1971), Homology: an unsolved problem Oxford University Press.
3. Rudolf Raff (1999), Larval homologies and radical evolutionary changes in early development, in B Hall (Ed.) Homology: The hierarchical basis of comparative biology, Academic Press; emphasis added.
4. Denis Duboule (1994), Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony, Development 1994 Supplement pp135-142. Duboule compared embryonic development with an 'egg-timer', but hourglass become the usual term.
5. Naoki Irie, Shigeru Kuratani (2014), The developmental hourglass model: a predictor of the basic body plan?, Development 141 pp4649-4655.
Image credits
Graphics are by David Swift unless stated otherwise.
The background image for the page banner is taken from an image by ★Kumiko★ – https://www.flickr.com/photos/kmkmks/27388394090/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=57660389
a. Ernst Haeckel's embryo drawings, redrawn by Romane (1892). https://commons.wikimedia.org/wiki/File:Haeckel_drawings.jpg In the Public Domain.
b. Taken from Figure 5 in Kenneth Wallace, Michael Pack (2003); Unique and conserved aspects of gut development in zebrafish, Dev. Biol. Mar 1;255(1):12-29. doi: 10.1016/s0012-1606(02)00034-9, Open Access.
c. Taken from Figure 5 in N Irie, N Satoh and S Kuratani (2018); The phylum Vertebrata: A case for zoological recognition; Zoological Letters 4(32):1-20; Open Access.
Page created October 2020; last modified March 2023.