Embryonic development of teleost fish

Zebrafish (typically 3-4 cm long) [a]
Teleosts (meaning ‘complete bone’) are the most abundant group of fish in terms of both number of species and individuals; they are what are generally understood by most people as ‘fish’. For various reasons which include ease of rearing, short development time, and a fairly transparent early embryo, most work on the embryonic development of teleosts has been done on the tropical freshwater zebrafish (Danio rerio), and the following description is based on this.
Egg and fertilisation
The egg comprises many membrane-bound yolk granules dispersed within cytoplasm, all within a shell (chorion) composed of various proteins and glycoproteins. There is a small hole (micropyle) in the chorion, near which the female polar body is situated (which identifies it as the animal pole), and through which spermatozoa can enter.
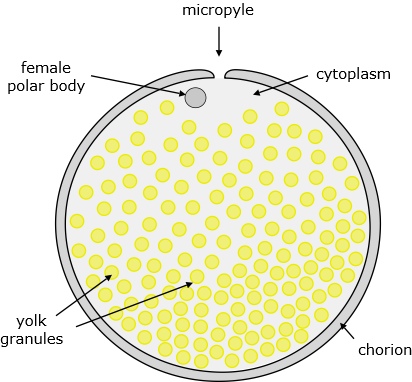
Figure 1. Zebrafish egg.
(In marine teleosts, the yolk is generally within one large granule rather than many small ones.)
On fertilisation, the chorion swells, the yolk granules concentrate at the vegetal pole, and the cytoplasm is displaced toward the animal pole where it forms a bulge, the blastodisc, which is where the first stages of embryo development take place.
Cleavage
Typical of eggs having a large yolk, cleavage is meroblastic, which means that the cell divisions are restricted to within the blastodisc and do not penetrate the yolk. The early cell divisions are very regular and synchronous: the first 5 divisions are all perpendicular to the plane of the blastodisc, but with the direction of cleavage alternating by 90°, to produce a regular array of 4 x 8 cells; the sixth division is then in the plane of the blastodisc, bisecting all of the existing cells, to give a total of 64 cells in two tiers of 32. These appear like a crown on top of the yolk cell. For cells in contact with the yolk, cell divisions are incomplete in the sense that membranes surrounding the new nuclei do not form completely, and the cells are cytoplasmically continuous with the yolk (and each other) to form the beginning of what is called a yolk syncitial layer (YSL). Further multiplication leads to a cap of cells occupying about a quarter of the yolk cell.

Figure 2. Cleavage in zebrafish up to formation of the blastula. [b]
Blastula
When there are about 128 cells (7 divisions after fertilisation), in what is known as the mid-blastula transition (MBT), they begin to differentiate:
- The outermost cells form a one-cell thick enveloping layer (generally known as the EVL). It is not part of the embryo, but remains an outer layer, becoming what is called the periderm which is shed when the embryo hatches from its shell. Nevertheless the EVL has a significant role in implementing gastrulation.
- As mentioned above, blastomeres adjacent the yolk do not fully separate from it. During the MBT, cells around the edge of the blastoderm fuse with the underlying yolk cell to produce a ring of YSL, which then spreads completely under the blastoderm (to form what is sometimes referred to as the ‘inner YSL’).
- The remaining cells of the blastoderm are known as the deep cells. It is these that become the embryo.
There is no blastocoel.

Figure 3. Zebrafish blastula.
Epiboly
All 3 layers begin to spread over the yolk cell, in a process known as epiboly. It appears that the driving force for epiboly is the EVL, showing that it is not merely a passive outer layer of cells.
A notable feature of epiboly is that the cap-shaped volume of deep cells becomes a layer of cells around the periphery, and along with this the upper surface of the yolk cell becomes dome-shaped (figure 4). By the time the layers have reached 30% towards the vegetal poly (30% epiboly) the deep layer is a uniform 4-cells thick, and it thins progressively (and uniformly throughout its extent) as it spreads around the rest of the yolk.
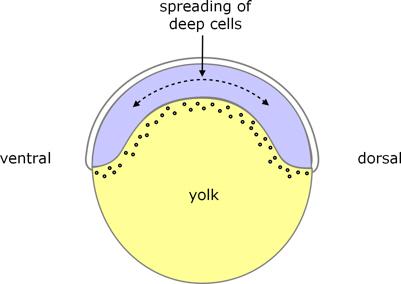
Figure 4. Zebrafish early epiboly.
During late blastula, some deep cells become compacted on the dorsal side, which is an early indication of the ventral-dorsal axis.
For a fate map of the blastula see here.
Gastrulation
Gastrulation begins when the spreading blastoderm, including the EVL and YSL, has reached around the ‘equator’ of the yolk, i.e. approximately 50% epiboly. At this stage, all around the margin of the blastoderm, some deep cells begin to turn under the edge of the blastoderm (involution) to form a new layer of tissue. The new layer is called hypoblast, the remaining outer layer is called epiblast (but note these are different from the epiblast and hypoblast of amniotes which form before gastrulation), and the initial thickening around the equator is called the germ ring. On the dorsal side, there is a group of 20-30 cells that do not involute, but move with the margin of the enveloping layer cells. At present their role is not known, but they end up as part of the tailbud.

Figure 5. Gastrulation in zebrafish.
As epiboly continues towards the vegetal pole, further cells involute, and there is an overall movement of hypoblast cells towards the animal pole, i.e. in the early stages the hypoblast moves in the opposite direction from the epiboly-related movement of the overlying epiblast.
However, as epiboly proceeds further, cells of both epiblast and hypoblast begin to converge towards the dorsal side, to form a thickening known as the embryonic shield. This shield, as well as extending further toward the vegetal pole as part of epiboly, also extends toward the animal pole.
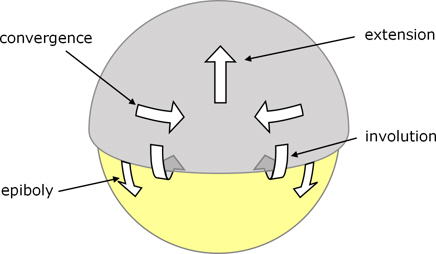
Figure 6. Zebrafish embryo viewed from the dorsal side to indicate cell movements during gastrulation.
The epiblast becomes the ectoderm, part of which forms the neural tube (see below), and is still enveloped by the EVL.
The hypoblast becomes endoderm and mesoderm: On the ventral and lateral edges of the blastoderm, the first cells that involute tend to become endoderm, and later involuting cells generally become mesoderm (although at the time of involution there is no obvious morphological difference). On the dorsal edge of the blastoderm, the first cells to involute form the prechordal plate, and these are followed by precursors of the notochord.
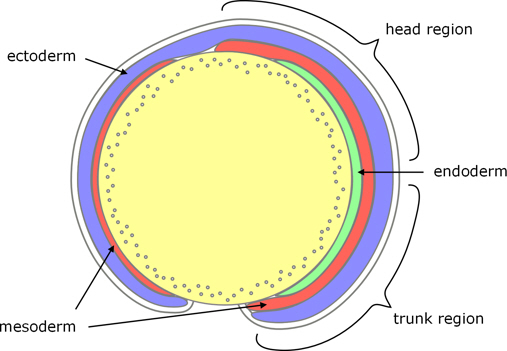
Figure 7. Zebrafish germ layers.
Eventually, epiboly extends over the whole of the yolk which, covered by mesoderm, becomes incorporated into the body of the embryo.
And in due course the embryonic shield also extends beyond both animal and vegetal poles, i.e. for a while it wraps around more than half of the yolk; later the tail straightens and moves away from the yolk (see figure 12).
Somites
At about the time that the embryonic shield stretches between the two poles, the mesoderm either side of the notochord (the paraxial mesoderm) starts to differentiate and segment into somites. These are blocks of paraxial mesoderm which form in pairs either side of the notochord. The first pair to arise is located between the head and trunk region, and subsequent pairs of somites form progressively toward the tail. Later, the somites contribute to the vertebrae.
Neural tube
It is during the segmentation period that the neural tube forms.
As indicated above, in the later stages of gastrulation both ectoderm and mesoderm thicken along the dorsal side to form the embryonic shield. The medial part of the ectoderm is the neural plate, and formation of the neural tube starts when the left and right sides of the neural plate converge toward the dorsal midline. The ectoderm first thickens, briefly forming a double layer of superficial and deep cells (not to be confused with the deep cells of the blastula stage, although derived from them).
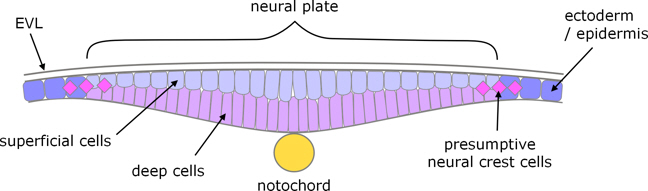
Figure 8. Zebrafish neural plate at the transient bilayer stage.
The deep cells then fold inwards along the midline, and the superficial cells intercalate with the deep ones, to form a wedge-shaped mass of cells called the neural keel. Consequently, the final neural tube is composed of cells originating from both superficial and deep cells.
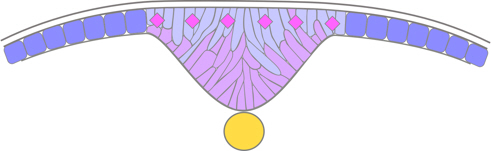
Figure 9. Zebrafish neural keel stage.
The keel then adopts a cylindrical or rod-like cross-section, with its cells aranged into right and left groups facing the midline, before separating from the ectoderm which reforms behind it.
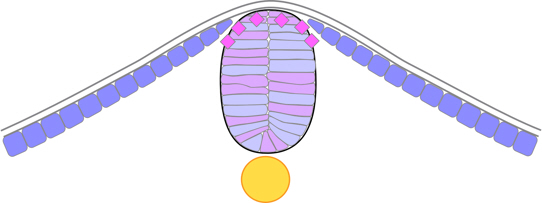
Figure 10. Zebrafish neural rod stage.
Only after this neural rod has formed does a cavity arise within it. A particularly interesting part of lumen formation in the zebrafish is that it entails cells on each side of the neural keel dividing to leave their daughter cells on both sides – called mirror-symmetric cell division. As a result, both sides of the neural tube receive equal contributions from both left and right sides of the neural plate (rather than each side of the neural tube being derived from cells of its respective side of the neural plate). Further, as the daughter cells become epithelial in nature to form the lumen, they must do so with opposite polarity, because they are on opposite sides of the lumen. The initial cavity is a slot which arises between the left and right halves, starting at the bottom (ventral) and developing towards the dorsal ectoderm, and this slot then widens to form the lumen of the neural tube. The lumen arises first in the head region (at about the 12-somite stage) and progresses caudally, being complete by about the 24-somite stage.
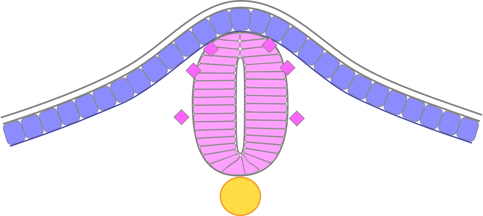
Figure 11. Zebrafish neural tube.
Neural crest cells
It will be apparent from the above description that the formation of the neural tube in zebrafish does not involve the occurrence of neural folds (as when the neural tube forms by fusion of neural ridges), so there are no neural crests. Nevertheless, presumptive ‘neural crest cells’ arise at the border of the neural plate, migrate to the dorsal part of the neural keel, and become evident as a distinct population of charactersitically neural crest cells between the reformed ectoderm and the newly formed neural tube, and subsequently migrate.
Formation of the gut
It is during the later stages of segmentation that the gut begins to form. It arises from within the endoderm on the dorsal side of the yolk. Initially some of the cells become organised into a radial pattern, and then a cavity forms within them to form the lumen of the digestive tract. This occurs first at the anterior end of the gut, followed by the hindgut, and then the midgut in-between.
A notable feature is that the anterior parts of the digestive tract (pharynx and oesophagus), and digestive organs (liver, pancreas) all arise independently of the digestive tract. They develop separately from distinct parts of the endoderm, and then join to the early digestive tract, rather than developing as extensions of the primitive gut (as in amniotes). In fact the progenitors of the liver and pancreas can be identified even before the onset of digestive tract morphogenesis.
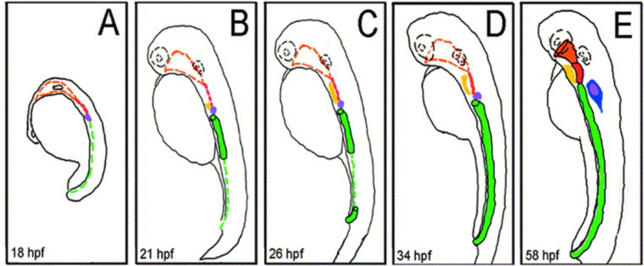
Figure 12. Schematic representation of zebrafish digestive system morphogenesis. (A) At 18 hpf, progenitors of the pharynx (orange), pancreas (purple), and gut (green) can be identified molecularly. (B) At 21 hpf, the foregut has formed and presumptive liver (yellow) progenitors can be identified. (C) At 26 hpf, the hindgut has formed and the liver progenitors have expanded. (D) At 34 hpf, the gut extends from the level of the finbuds to the developing anorectum. The pharynx and esophagus are undeveloped at this stage. (E) At 58 hpf, the pharynx (orange), esophagus (red), and developing intestine (green) are contiguous. Cells within the developing exocrine pancreas (blue) are also now recognizable. hpf = hours post fertilisation [c]
Only in teleosts does the gut form by cavitation within the endoderm.
Image credits
Graphics are by David Swift unless stated otherwise.
Background image for the page banner is by DrKontogianniIVF from www.needpix.com/photo/download/674083/embryo-ivf-icsi-infertility-fertility-free-pictures-free-photos-free-images-royalty-free.
a. Zebrafish. From goodfreephotos.com/animals/fish/zebrafish-danio-rerio.jpg.php Available under the CC0 / Public Domain License.
b. Cleavage in zebrafish. Author: Gilbert, SF. From https://en.m.wikipedia.org/wiki/File:Cleavage.png licensed under the Creative Commons Attribution-Share Alike 4.0 International license. Letters against each of the images have been blanked out.
c. Reproduced from Figure 5 in Kenneth Wallace, Michael Pack (2003); Unique and conserved aspects of gut development in zebrafish, Dev. Biol. Mar 1;255(1):12-29. doi: 10.1016/s0012-1606(02)00034-9, Open Access.
Page created October 2020.